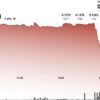
ImmunityBio (IBRX) witnessed a remarkable surge of 19.8% in its stock price during premarket trading on Tuesday. This surge follows a constructive meeting with the FDA regarding the company”s bladder cancer treatment, ANKTIVA.
The FDA”s feedback was pivotal as the agency encouraged ImmunityBio to submit additional information to support a resubmission of its supplemental Biologics License Application. Notably, the FDA did not mandate new clinical trials, a significant relief for the company.
ImmunityBio intends to act swiftly, committing to deliver the requested information within a 30-day timeframe. This application is aimed at expanding the label for ANKTIVA, which is already approved for certain bladder cancer patients. The treatment received FDA approval in April 2024 for individuals diagnosed with carcinoma in situ, with or without papillary tumors.
Long-term clinical data published in The Journal of Urology further bolsters the treatment”s efficacy. The study indicated an impressive 96% bladder cancer-specific survival rate at three years, alongside a bladder preservation rate exceeding 80% within the same timeframe. These findings came from a study tracking 80 patients, with median outcomes still pending.
The regulatory journey has not been without challenges. Earlier, in May 2025, the FDA issued a Refusal to File letter concerning the supplemental application related to papillary tumors. However, recent developments signal a more favorable trajectory.
Internationally, ANKTIVA has also secured approvals beyond the United States, including in the United Kingdom and Saudi Arabia, with the European Union granting conditional approval. This broadens the treatment”s potential patient demographic across various markets.
Richard Adcock, President and CEO of ImmunityBio, expressed gratitude for the regulatory process and reiterated the company”s dedication to delivering this critical therapy to patients lacking approved alternatives.
Wall Street analysts reacted positively to the FDA”s feedback, with Piper Sandler analyst Edward Tenthoff raising his price target for ImmunityBio from $5.00 to $7.00, maintaining an Overweight rating on the stock. This reflects confidence in the company”s regulatory path moving forward.
This stock movement comes on the heels of a strong week, where shares had previously more than doubled in value before the latest premarket gain.
As ImmunityBio prepares to submit the additional data within the stipulated timeline, stakeholders will be keenly observing the developments, particularly in the context of the ongoing advancements in cancer treatment therapies.