Anavex Life Sciences Corp. (NASDAQ: AVXL) faced a dramatic decline in stock value on November 14, 2025, as shares plummeted approximately 50% in premarket trading. This significant downturn followed a negative vote from the European Medicines Agency“s Committee for Medicinal Products for Human Use (CHMP) regarding the company”s Alzheimer”s drug candidate, blarcamesine.
This rejection marks a critical setback for the clinical-stage biopharmaceutical firm, jeopardizing its plans for European market entry and raising concerns about the future of its primary therapeutic program.
The adverse decision from the CHMP came after an oral hearing related to Anavex”s Marketing Authorization Application (MAA) for blarcamesine, a once-daily oral treatment aimed at early Alzheimer”s disease. The EMA had initially accepted the MAA in December 2024, making this recent reversal particularly impactful on the company”s regulatory strategy.
The CHMP is set to issue a formal opinion regarding the application in its upcoming December meeting. Following this, Anavex plans to seek a re-examination of the decision from a different panel of reviewers.
Despite the setback, Dr. Juan Carlos Lopez-Talavera, Anavex”s Head of Research and Development, underscored the importance of the dialogue with the CHMP, stating that the discussions provided “valuable education and engagement” for the blarcamesine program. The company continues to promote the drug”s potential benefits, including its oral delivery method, unique mechanism of action, and a safety profile that reportedly does not necessitate routine MRI monitoring, which could differentiate it from existing treatments for Alzheimer”s.
In a related update, the U.S. Food and Drug Administration has encouraged Anavex to request a meeting to discuss the results of its Alzheimer”s disease clinical trials, indicating that while challenges exist in Europe, there remains a pathway for potential authorization in the United States, the largest pharmaceutical market globally.
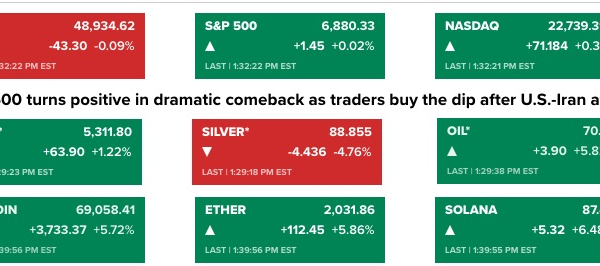
As market reactions unfolded, AVXL shares traded at $3.02 in premarket activity as of 8:44 AM EST on November 14, 2025, reflecting a decline of $2.67 or 46.92% from the prior close of $6.90. The stock had already experienced a 17.54% drop, closing at $5.69 during the regular trading hours on November 13, culminating in a total decline of roughly 56% over two days.
This steep drop pushed the stock to a 52-week low of $5.10, resulting in a substantial loss of shareholder value and reducing the company”s market capitalization to approximately $488.74 million. The biotechnology sector is known for its volatility, and AVXL has exhibited significant sensitivity to regulatory updates. Year-to-date, the stock has lost 47.02%, starkly underperforming the S&P 500, which has gained 14.55% during the same timeframe.
Trading volume surged dramatically to 8,587,428 shares, more than six times the average volume of 1,357,682, indicating intense selling pressure and investor trepidation regarding the company”s future prospects. Despite the current turmoil, some analysts maintain a bullish outlook for AVXL, with an average price target of $44.00 and a high estimate of $46.00, suggesting potential upside of nearly 673% from the current premarket levels. However, these projections were made prior to the CHMP decision and may be revised as analysts reassess the company”s regulatory and commercial trajectory in light of the recent setback.